Abstract
Background:Most patients with relapsed/refractory follicular lymphoma (r/r FL) remain incurable and eventually relapse or progress. Previously, initial findings of the pivotal Phase 2 RELIANCE study (NCT04089215) demonstrated a high response rate and low rate of chimeric antigen receptor (CAR) T-associated toxicity with relmacabtagene autoleucel (relma-cel) treatment in heavily pretreated patients with r/r FL. Here we present the 6-month follow-up results of efficacy, safety, and pharmacokinetics (PK).
Methods:Eligible patients with r/r FL had histologically confirmed follicular lymphoma grade (Gr) 1, 2, or 3a, with relapsed/refractory disease after ≥2 prior lines of therapies containing anthracycline and anti-CD20 agents (e.g. rituximab) or auto-HSCT. Patients were randomized to receive a single intravenous infusion of autologous CAR+T cells at a dose of 100×106 or 150×106, following lymphodepletion chemotherapy (fludarabine 25 mg/m2 & cyclophosphamide 250 mg/m2 daily×3 days).
Patients were evaluated for efficacy (Lugano criteria, 2014), safety (cytokine release syndrome [CRS] by Lee 2014, and all other events by CTCAE v4.03,) and PK (quantitative polymerase chain reaction [qPCR]). The primary endpoint was 3-month complete response rate (CRR). Key secondary endpoints included 3-month objective response rate (ORR), duration of response (DOR), progression-free survival (PFS), overall survival (OS), treatment-emergent adverse event (TEAE) profile, and PK.
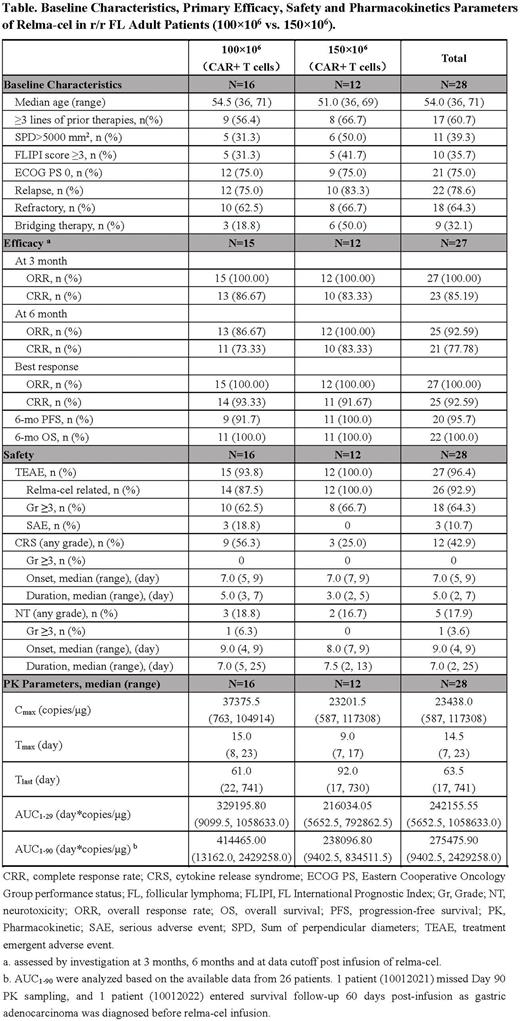
Results: At data cut-off (Dec 17, 2021), 28 patients were enrolled and all completed 6-month follow-up. Among them, 16 patients received relma-cel of 100×106 CAR+ T cells, and 12 patients received 150×106 CAR+ T cells. The median age was 54.0 years (range, 36-71) and 50% were male. 21 patients (75.0%) had ECOG 0. Histology included FL Gr 1 (n=1), Gr 2 (n=13), Gr 3a (n=10), not specified (n=4). 11 patients (39.3%) had a high tumor burden with SPD >5000 mm2. 10 patients (35.7%) had FLIPI2 scores ≥3. 17 patients (60.7%) had received ≥3 lines of prior therapies. 18 patients were refractory to and 22 patients had relapsed from prior therapy. 9 patients received bridging chemotherapy.
Among the 27 efficacy evaluable patients (1 patient was excluded from mITT due to the co-occurrence of hypofractionated adenocarcinoma of the stomach), the primary endpoint 3-month CRR was 85.19% (95% confidence interval [CI], 66.27-95.81). 3-month ORR was 100% (95% CI, 87.23-100.00). At the 6-month follow-up, 92.59% of patients (95%CI, 75.71-99.09) were in response with 77.78% of patients (95%CI, 57.74-91.38) in complete response. At data cut-off, the best ORR achieved was 100% (95%CI, 87.23-100.00) with CRR of 92.59% (95%CI, 75.71-99.09). With a median follow-up of 11.7 months, median DOR, PFS, and OS have not been reached. 6-month PFS and OS rates were 95.7% and 100%, respectively. Responses were similar across 100×106 and 150×106 dose groups, as shown in the table.
Of 28 treated patients, 18 (64.3%) patients had Gr ≥3 TEAEs, the most common were neutropenia (39.3%), leukopenia (25.0%), and lymphopenia (17.9%). The incidence of CRS and neurotoxicity (NT) was 42.9% and 17.9%, respectively. The median time to CRS onset was 7 days (range, 5-9) with a median duration of 5 days (range, 2-7). No Gr ≥3 CRS was observed. The median time to NT onset was 9 days (range, 4-9) with a median duration of 7 days (range, 2-25). Only 1 patient experienced Gr ≥3 NT. To manage CRS and/or NT, 5 patients received tocilizumab or corticosteroids. No deaths occurred. qPCR assay showed that the CAR transgene was firstly detectable from Day 4 to 15 after infusion, and time to peak CAR-T cell expansion was 7 to 23 days. 7 patients still had CAR-T detectable on Day 180. The table presented similar safety profiles and PK characteristics across 100×106 and 150×106 dose groups.
Conclusions: With 11.7 months of median follow-up, relma-cel demonstrated remarkable clinical responses achieving high rates of CR and OR, and a manageable safety profile in the phase 2 study of CAR T cell therapy in r/r FL. Updated safety and efficacy data with a longer follow-up will be presented.
Disclosures
Tian:JW Therapeutics: Current Employment. Huang:JW Therapeutics: Current Employment. Zhou:JW Therapeutics: Current Employment. Ma:JW Therapeutics: Current Employment. Yang:JW Therapeutics: Current Employment. Qin:JW Therapeutics: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal